1. What Happened?
On August 29, 2025, Korea BioNics secured a partial victory in the first trial of the Vienox lawsuit. The key outcome was the lifting of the provisional injunction against the manufacturing of Vienox for export. While the court upheld the recall and disposal order, the cancellation of the manufacturing suspension holds significant weight for the company.
2. Why Does it Matter?
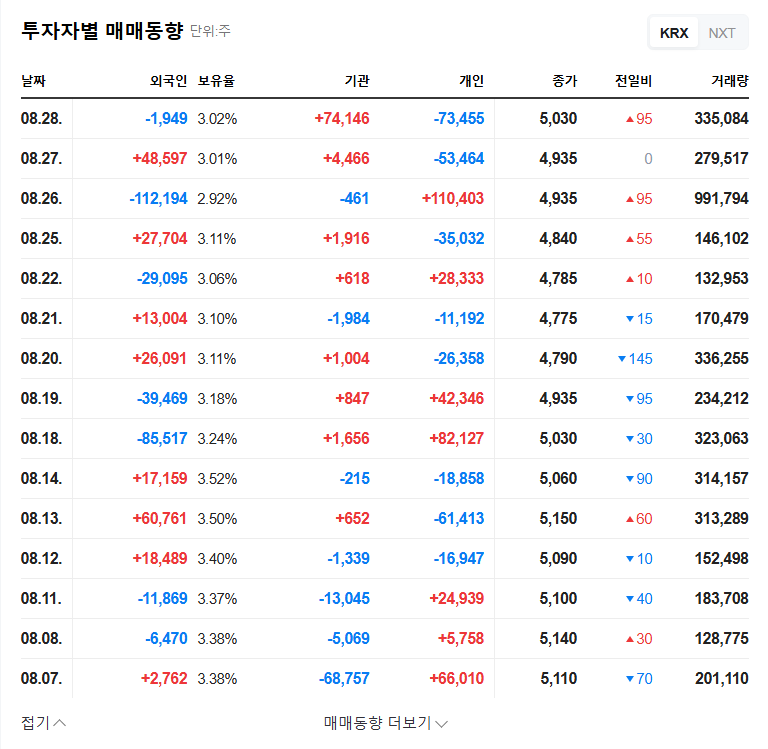
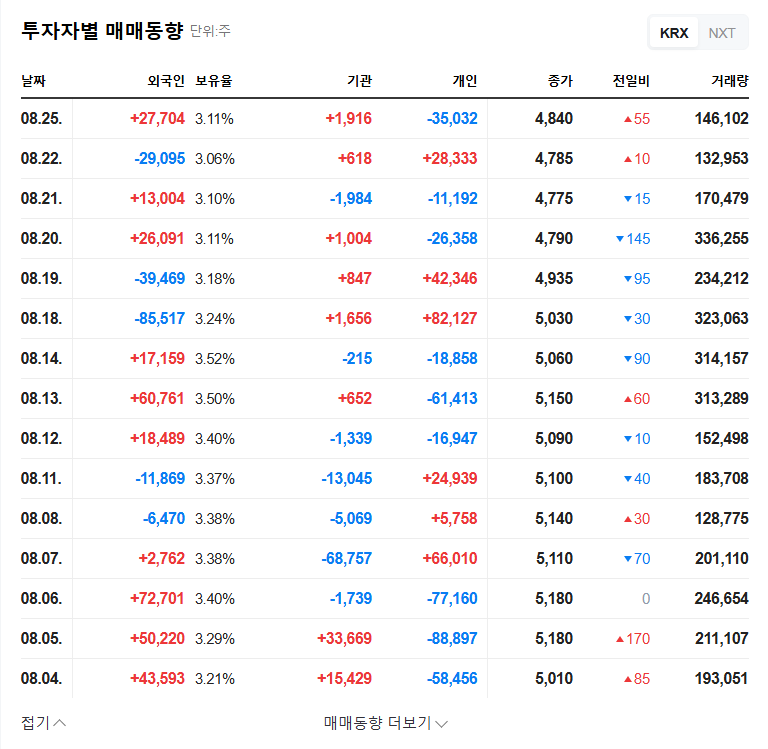
A substantial portion of Korea BioNics’ revenue stems from exports. This ruling opens up the possibility of resuming manufacturing and sales of Vienox for export, which could positively impact the company’s financial stability and growth prospects. Avoiding the worst-case scenario for business operations is also crucial.
3. What’s Next?
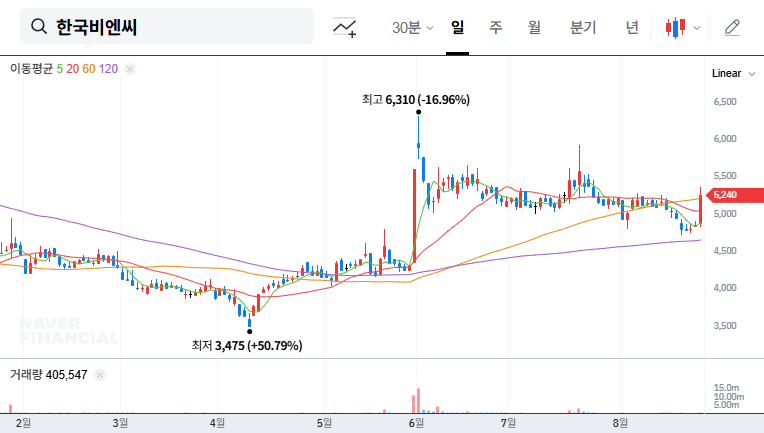
This ruling is expected to have a positive short-term impact on Korea BioNics’ stock price. The resolution of business uncertainty and the potential for continued sales activity can improve investor sentiment. However, potential risks remain, such as the possibility of an appeal and the potential for costs related to the recall and disposal order.
4. What Should Investors Do?
While short-term momentum for a price increase exists, long-term investment requires caution. Careful monitoring of the appeal process, subsidiary issues, and macroeconomic volatility is crucial for informed investment decisions. A thorough fundamental analysis, including the company’s business portfolio, market competitiveness, and financial health, is essential.
Frequently Asked Questions
What is the impact of this ruling on Korea BioNics’ stock price?
A positive short-term impact is expected, but long-term effects depend on the appeal outcome and market conditions.
What is Vienox?
Vienox is a botulinum toxin type A product.
What are Korea BioNics’ main businesses?
Korea BioNics manufactures and sells medical devices for aesthetic plastic surgery and anti-aging, including HA fillers, botulinum toxins, wound dressings, and surgical medical devices.