What Happened?
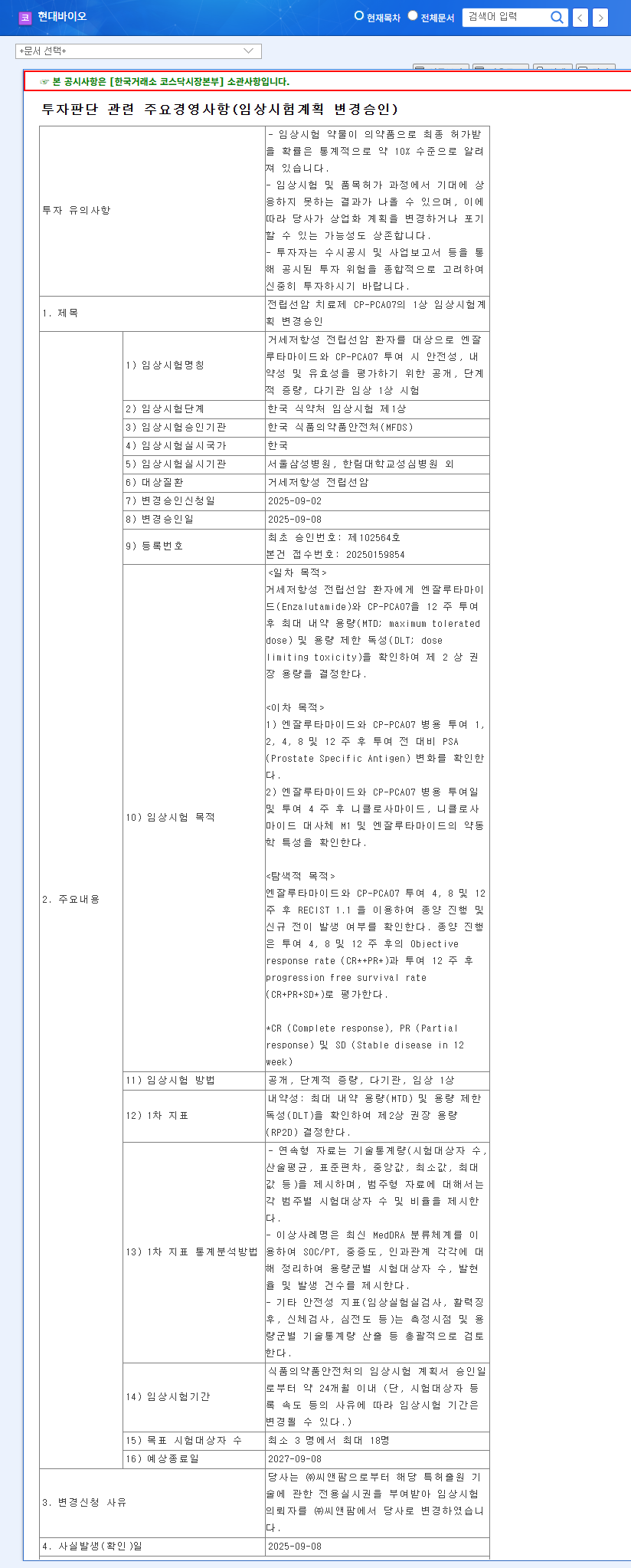
On September 8, 2025, Hyundai Bioscience received approval to amend the Phase 1 clinical trial plan for its prostate cancer drug, CPPCA07. This trial aims to evaluate the safety, tolerability, and efficacy of CP-PCA07 in combination with enzalutamide in patients with castration-resistant prostate cancer.
Why is This Approval Important?
Approval for the Phase 1 clinical trial amendment is a crucial first step in drug development. This is the first stage in confirming the safety and efficacy of CPPCA07 and serves as a stepping stone for future Phase 2 and 3 clinical trials. Specifically, this trial outlines clear objectives such as determining the Maximum Tolerated Dose (MTD) and Dose Limiting Toxicity (DLT), providing greater visibility into the potential for success.
What’s Next?
Positive Phase 1 results could drive an increase in Hyundai Bioscience’s corporate value. However, it’s crucial to understand that clinical trials are lengthy and costly processes. Investors should approach this with a long-term perspective rather than reacting to short-term stock fluctuations. It’s also important to watch the clinical progress and potential synergies with other pipeline developments, such as Hyundai Bioscience’s COVID-19 treatment and pancreatic cancer drug.
Investor Action Plan
- • Pay close attention to the upcoming Phase 1 clinical trial results.
- • Analyze the development status of other pipelines and potential synergistic effects.
- • Monitor financial soundness and potential for technology transfer.
- • Maintain a long-term investment perspective.
Frequently Asked Questions
What is CPPCA07?
CPPCA07 is a prostate cancer drug candidate being developed by Hyundai Bioscience.
When will the Phase 1 trial begin?
The exact start date of the clinical trial has not yet been disclosed.
What are the key investment considerations?
Careful investment decisions are needed, considering the uncertainties inherent in drug development.