The upcoming ABL Bio Inc. IR event is a critical juncture for investors, promising to unveil key insights into the company’s trajectory. Scheduled for November 17, 2025, at 1:00 PM (KST), this event will shed light on the company’s recent successes and future ambitions. For anyone holding or considering an investment in ABL Bio Inc. (298380), this deep-dive analysis will explore the impressive financial growth, groundbreaking technology platforms, and the potential market impact, helping you formulate a robust investment strategy.
We will dissect the fundamentals, evaluate external economic factors, and outline potential scenarios—both positive and negative—that could influence the stock price following the presentation. This comprehensive guide provides the crucial context you need to make informed decisions.
Why This IR Event is a Game-Changer for Your ABL Bio Inc. Investment Strategy
An Investor Relations (IR) event is more than just a presentation; it’s a direct line of communication between a company’s leadership and its stakeholders. For a dynamic biotech firm like ABL Bio Inc., this event is pivotal. It offers a platform to showcase progress, clarify complex R&D milestones, and instill confidence in the market. The information revealed could serve as a powerful catalyst for the company’s valuation and is a key component for any ABL Bio Inc. investment strategy.
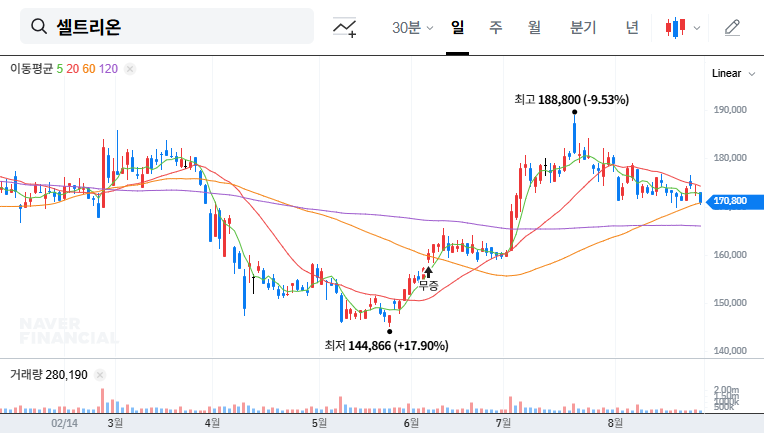
Financial Fortress: Analyzing the Q3 2025 Surge
ABL Bio Inc.’s Q3 2025 report was nothing short of spectacular. The company posted revenue of KRW 79.35 billion, a staggering 226% year-over-year increase. This growth was not incidental; it was primarily fueled by KRW 73.17 billion in technology transfer revenue from a major UK client. This demonstrates that ABL Bio’s business model of developing and licensing its technology is firing on all cylinders. Furthermore, improved liquidity, with cash reserves soaring 121% to KRW 124.36 billion, signals a strong financial foundation for future R&D initiatives, as confirmed in their Official Disclosure on DART (Source).
The successful monetization of its technology transfer business is a clear indicator that ABL Bio Inc. is transitioning from a development-stage biotech to a commercially viable enterprise. This shift is a key reason for the heightened market anticipation surrounding this IR event.
The Technology Powerhouse: A Closer Look at Grabody™ and Key Pipelines
At the heart of ABL Bio’s innovation is its proprietary bispecific antibody platform, Grabody™. This technology allows a single antibody to target two different disease mechanisms simultaneously, opening doors for more effective treatments in oncology and immunology. You can learn more about bispecific antibody technology in our detailed guide. Key pipelines built on this platform include ABL001, ABL111, and promising ADC candidates.
Even more compelling is the ‘Grabody-B’ platform, a Blood-Brain Barrier (BBB) shuttle technology. The BBB is a major hurdle in treating brain diseases, and Grabody-B is designed to overcome it, potentially revolutionizing treatments for neurodegenerative conditions like Alzheimer’s and Parkinson’s. The progress of the ABL301 pipeline, which utilizes this tech, will be a focal point of the IR event.
Potential Stock Impact: Bull vs. Bear Scenarios
The content shared at the ABL Bio Inc. IR event will likely create significant market movement. Investors should be prepared for various outcomes.
The Bull Case: Positive Catalysts
- •Positive R&D Updates: Favorable clinical trial data or progress announcements for key pipelines like ABL001 or ABL301 could significantly boost investor confidence and drive the 298380 stock price upward.
- •Strong Future Guidance: A confident outlook for H2 2025 and beyond, including new partnership hints or technology transfer deals, would reinforce the growth narrative.
- •Management Clarity: A transparent and compelling presentation on cost control, R&D efficiency, and risk management will build long-term trust with institutional investors.
The Bear Case: Potential Headwinds
- •Underwhelming News: If the presentation fails to deliver new, impactful information beyond what is already known, the market may react with disappointment, leading to a short-term price correction.
- •Unexpected Setbacks: Any mention of unforeseen delays in clinical trials or challenges with platform development, however minor, could be perceived negatively in the high-stakes biotech sector.
- •Macroeconomic Concerns: Management’s commentary on the impact of high interest rates or currency fluctuations could spook investors if not addressed with a clear mitigation strategy. According to industry analysis from sources like high-authority financial news sites, biotech funding is sensitive to these factors.
Investor Action Plan & Recommendations
Given the high-impact nature of this event, a proactive approach is recommended. A thorough ABL Bio Inc. analysis should form the basis of your actions.
- •Monitor Closely: Pay keen attention to the live presentation and, most importantly, the Q&A session, where unplanned, crucial details often emerge.
- •Adopt a Long-Term View: The biotech industry is inherently volatile. Base your investment on the long-term potential of the Grabody™ platform and the company’s pipeline, not just on a single day’s stock movement.
- •Assess Your Portfolio: Depending on the outcomes, be prepared to adjust your portfolio. Exceptionally positive news might justify increasing your position, while cautious news might warrant a re-evaluation.
In conclusion, the ABL Bio Inc. IR event is far more than a corporate formality; it’s a strategic communication that will set the tone for the company’s market performance in the coming months. By understanding the fundamentals and potential outcomes, investors can navigate the post-event landscape with greater confidence.