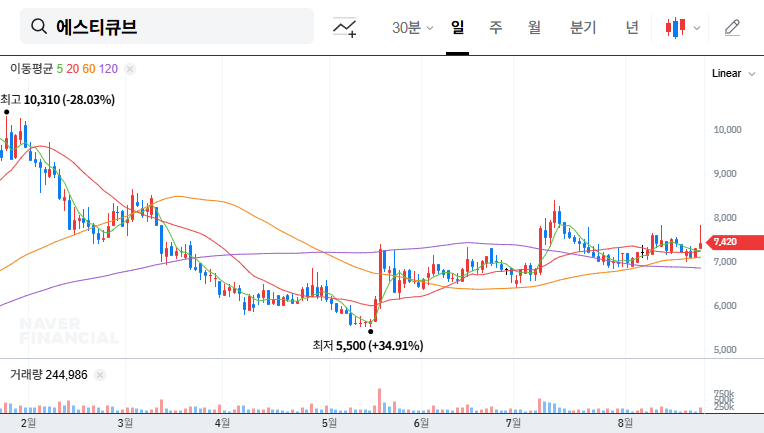
What Happened?
On August 25, 2025, STCube announced the voluntary withdrawal of its Phase 1b/2 clinical trial for hSTC810, an immunotherapy drug for relapsed or refractory extensive-stage small cell lung cancer (R/R ES-SCLC). This trial had previously received approval from both the Korean MFDS and the US FDA.
Why the Halt?
While the official reason remains unclear, the voluntary withdrawal after FDA approval suggests a potentially serious issue such as toxicity, lack of efficacy, or a strategic shift in development. This falls significantly short of market expectations and raises doubts about the drug’s potential success.
What Does This Mean for Investors?
-
Short-Term Impact:
- Potential for a sharp decline in stock price
- Negative investor sentiment
-
Long-Term Impact:
- Weakened drug development competitiveness
- Erosion of investor confidence
- Potential difficulties in raising capital
Although STCube’s previous efforts to enhance transparency by correcting past business reports were seen as positive, this clinical trial halt significantly offsets those gains. Given STCube’s already precarious financial situation, the impact of this event is likely to be substantial.
What Should Investors Do?
Investors should closely monitor STCube’s future R&D strategies, funding plans, and management’s communication. It is crucial to consider the possibility of further setbacks and exercise caution in investment decisions. Referencing the company’s official announcements and expert analysis is vital for effective risk management.
FAQ
Q: Why did STCube halt the clinical trial?
A: The exact reason is undisclosed, but the voluntary withdrawal post-FDA approval suggests a serious issue, potentially related to drug safety, efficacy, or a strategic change in development.
Q: What is the outlook for STCube’s stock price?
A: The stock price is expected to face significant downward pressure in the short term. The long-term outlook depends on the company’s response and further information releases.
Q: What actions should investors take?
A: Investors should closely monitor the company’s future R&D strategy and financial situation while awaiting further information. It’s essential to avoid impulsive investment decisions and rely on expert analysis for informed decision-making.

Leave a Reply